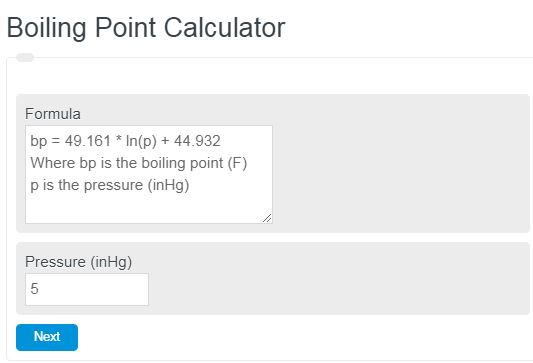
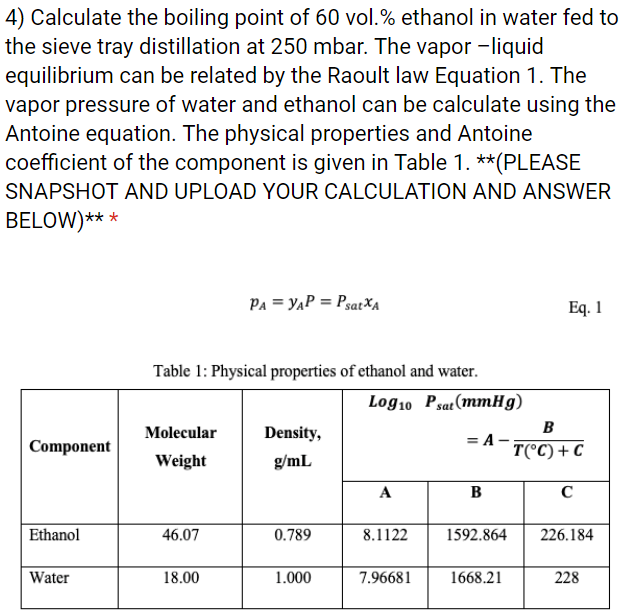
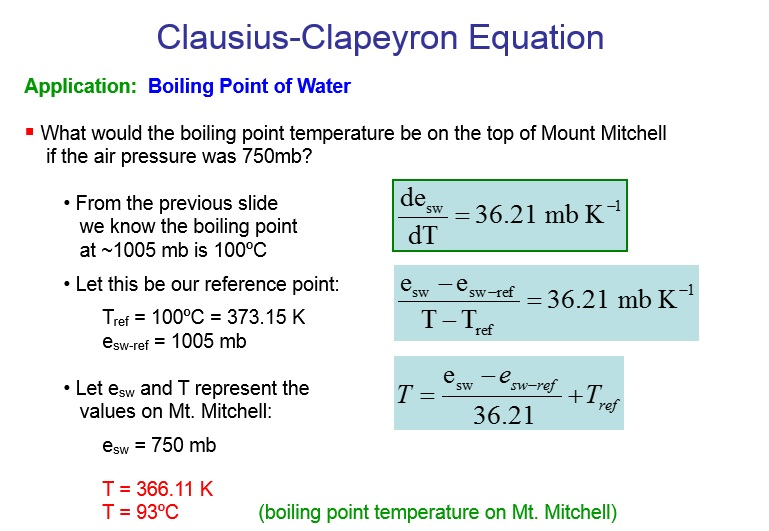
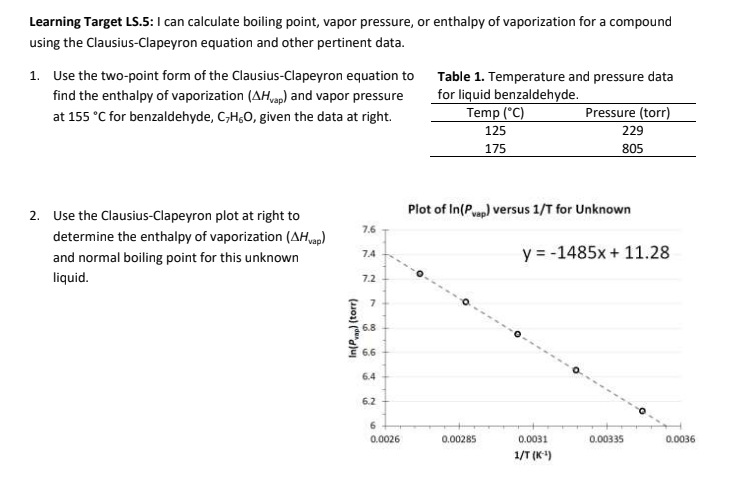
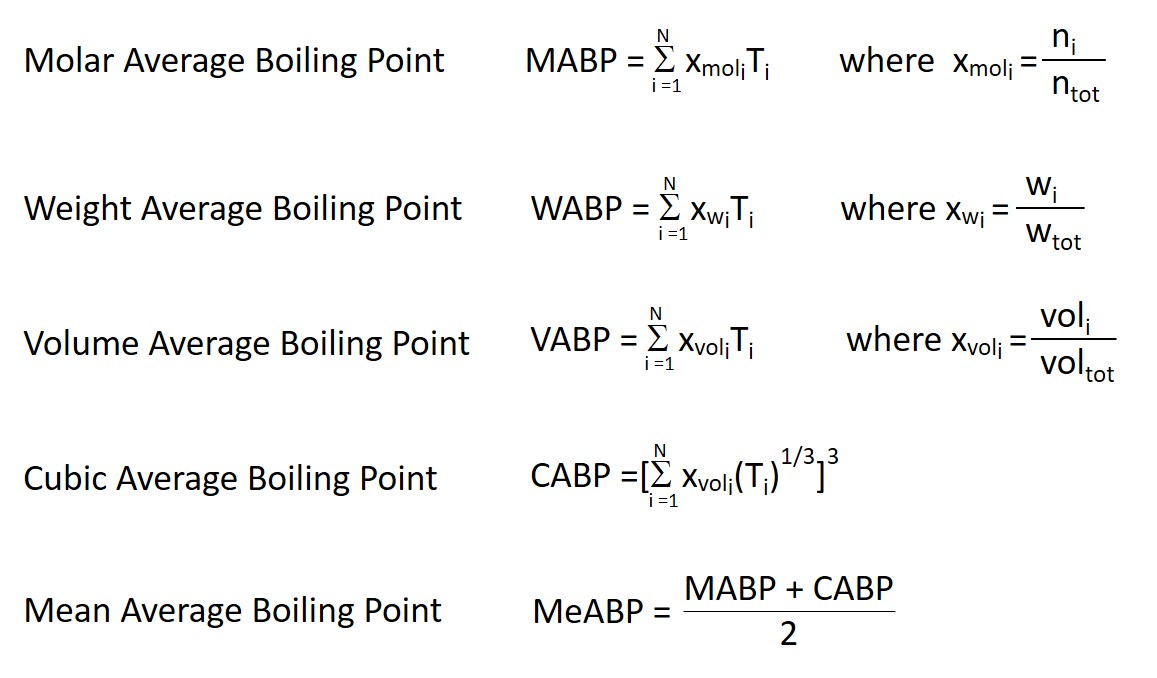
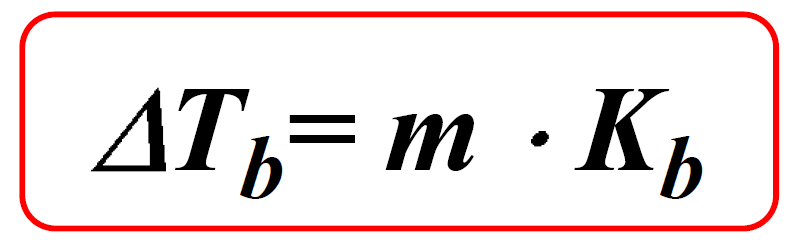Calculate the freezing point and the boiling point at 1 atmosphere of a solution containing 30 g cane sugar (molecular mass 342 ) and 150 g water.Given : Kb = 0.513 and Kf = 1.86
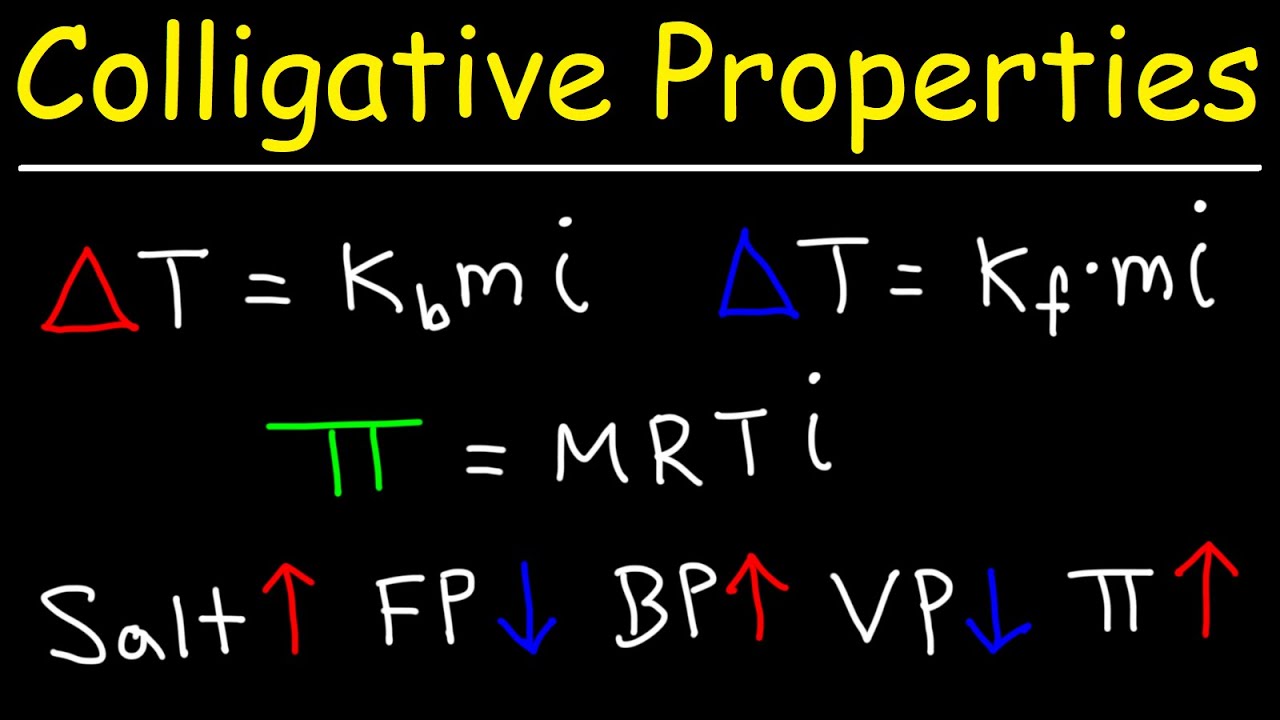
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure - YouTube

In a mountainous location, the boiling point of pure water is found to be 95 degrees Celsius. How many grams of sodium chloride much be added to 1 kg of water to

Determine the normal boiling point in K of a substance whose vapor pressure is 55.1 mmHg at 23.2^o C and has a ?H_vap of 32.1 kJ/mol. | Homework.Study.com
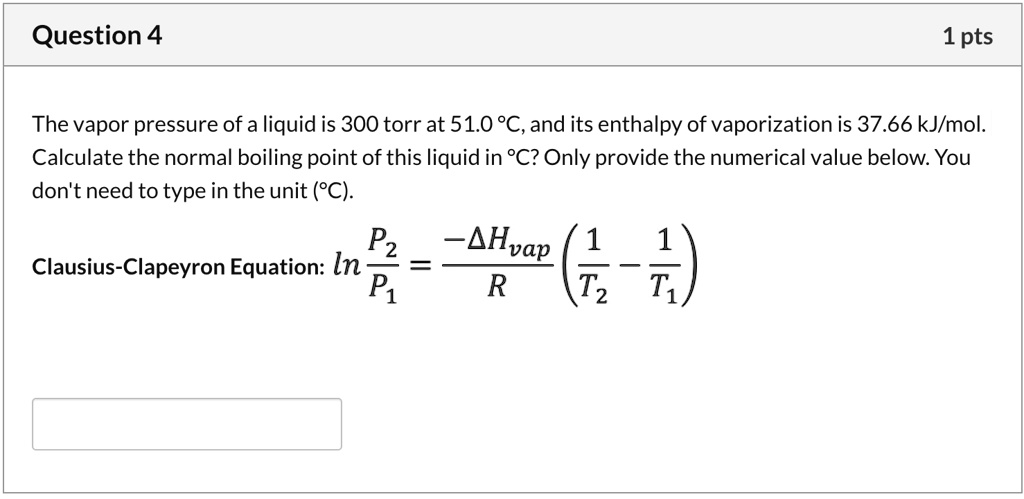
SOLVED: Question 4 1pts The vapor pressure of a liquid is 300 torr at 51.0 *C,and its enthalpy of vaporization is 37.66 kJlmol. Calculate the normal boiling point of this liquid in *

Boiling Point Elevation and Freezing Point depression - Example 2 ( Video ) | Chemistry | CK-12 Foundation