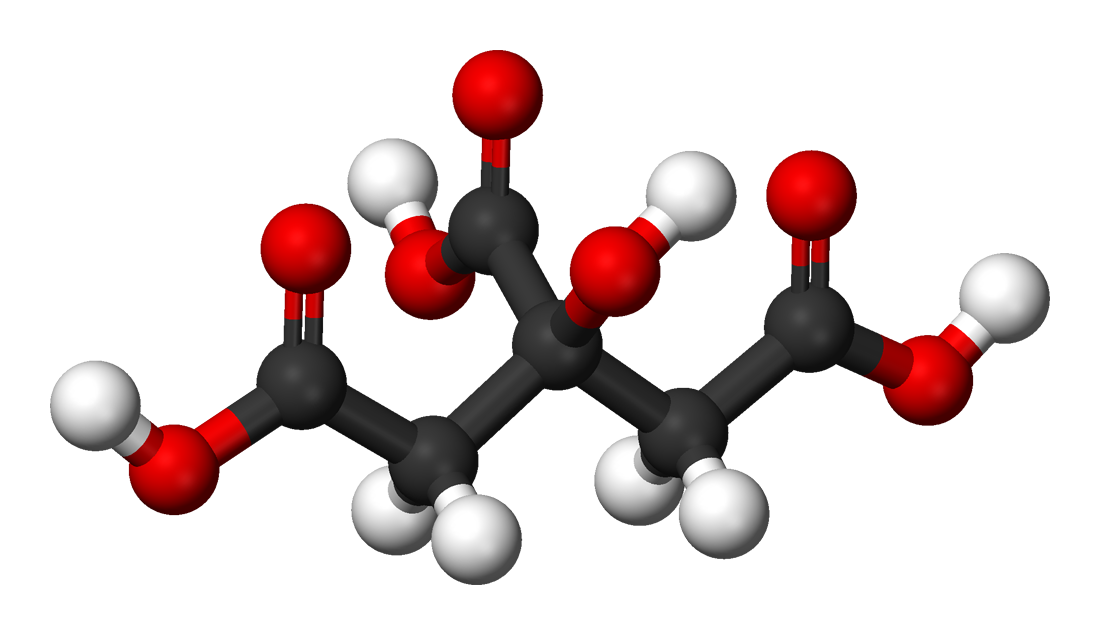
Citric acid is a triprotic acid with Ka values of 3.14, 5.95, and 6.39. Estimate the pH of a 0.15 M solution of a monosodium salt. | Homework.Study.com

SOLVED: The pH of the 0.5% w/v citric acid solution is 4. Citric acid is a weak acid, pka=4.76. (Mol wt: 192g/mole) a. Calculate the % ionization of diluted citric acid solution
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://i.ytimg.com/vi/AufT6_CoFWY/maxresdefault.jpg)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]
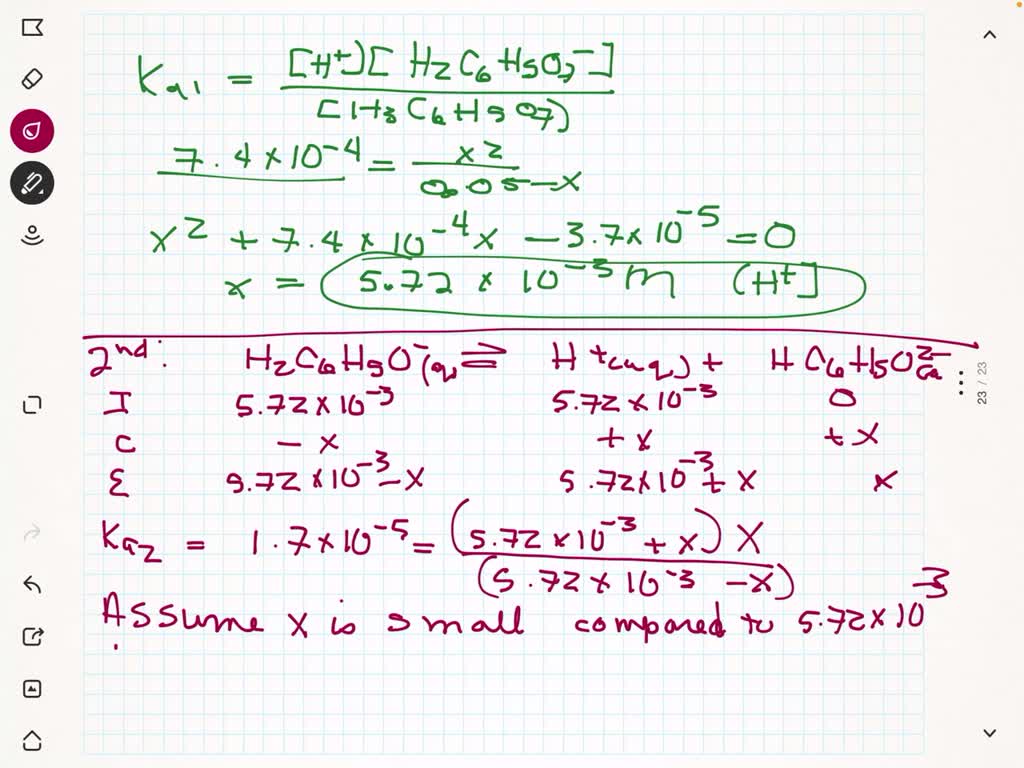
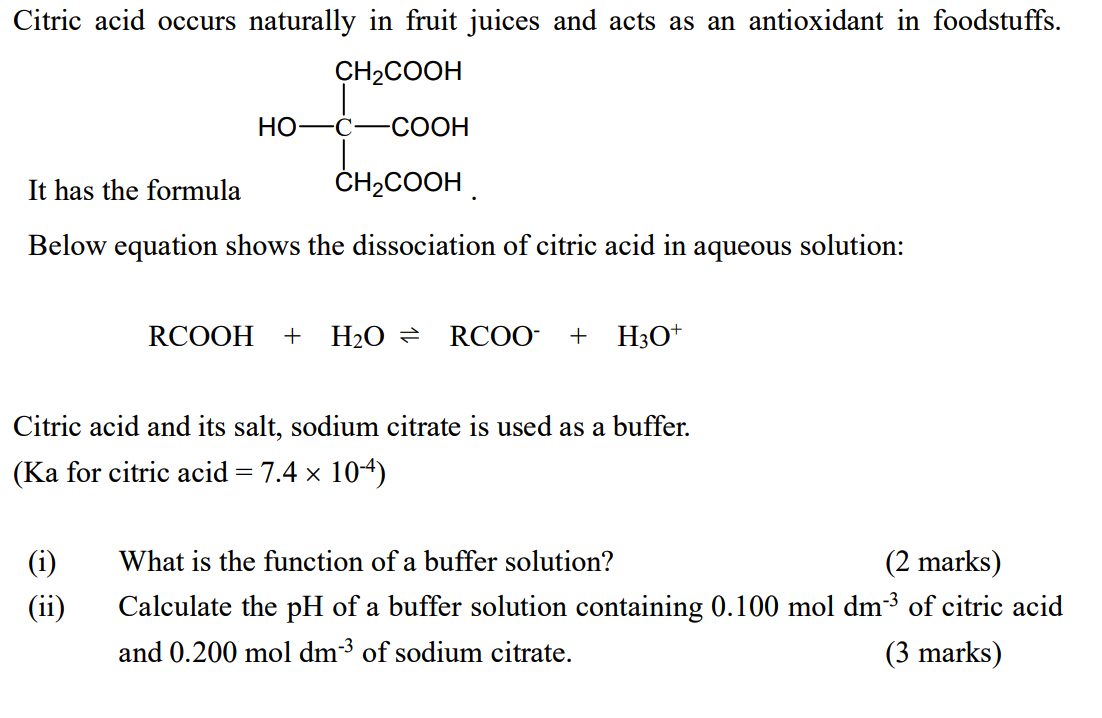
SOLVED:Citric acid, which is present in citrus fruits, is a triprotic acid (Table 16.3). Calculate the pH and the citrate ion (C6 H5 O7 ^3) concentration for a 0.050 M solution of

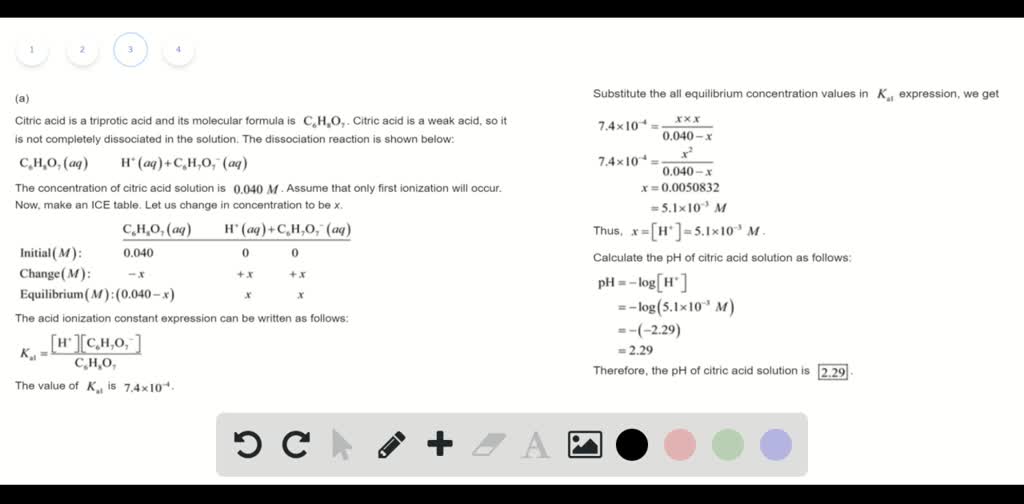
Calculate the pH and the equilibrium concentrations of H2C6H5O7-, HC6H5O72-, and C6H5O73- in a 0.1380 M aqueous citric acid solution. For H3C6H5O7, Ka1 = 7.4 x 10-3, Ka2 = 1.7 x 10-5, and Ka3 = 4.0 x 10-7. | Homework.Study.com

Calculate the pH and the equilibrium concentrations of H2C6H5O7-, HC6H5O72-, and C6H5O73- in a 0.1380 M aqueous citric acid solution. For H3C6H5O7, Ka1 = 7.4 x 10-3, Ka2 = 1.7 x 10-5, and Ka3 = 4.0 x 10-7. | Homework.Study.com

acid, hydrochloric acid, and citric acid. Dilution factor is calculated... | Download Scientific Diagram
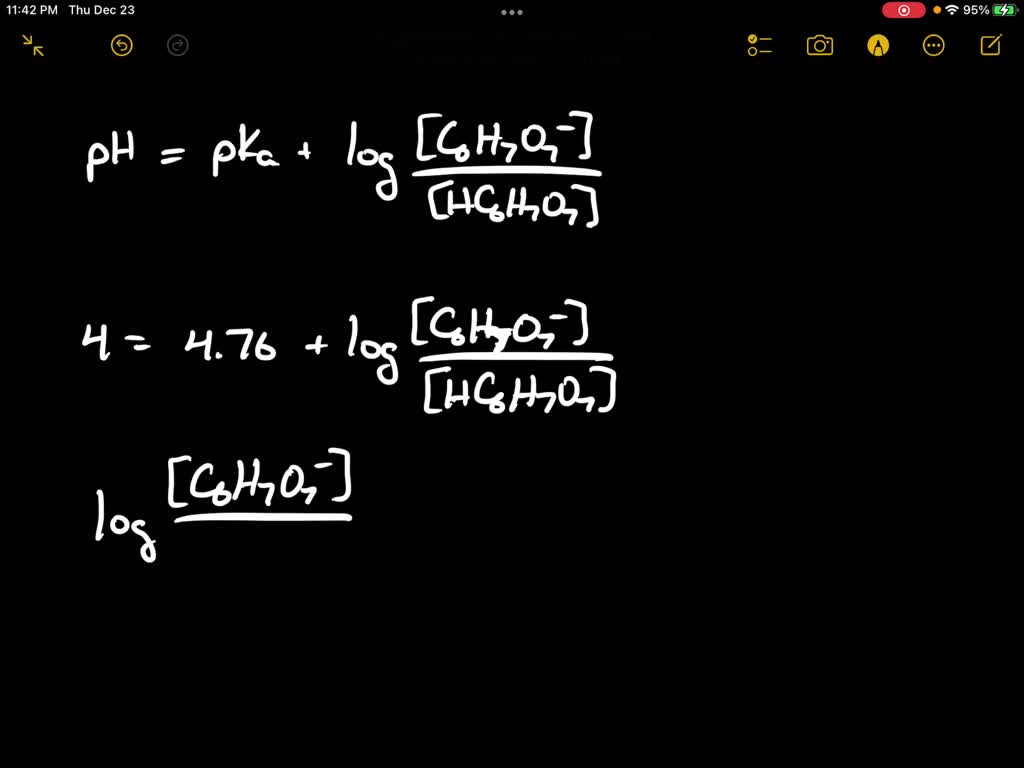
SOLVED: The pH of the 0.5% w/v citric acid solution is 4. Citric acid is a weak acid, pka=4.76. (Mol wt: 192g/mole) a. Calculate the % ionization of diluted citric acid solution

Lemon juice normally has a `pH` of `2`. If all the acid the lemon juice is citric acid and there are - YouTube
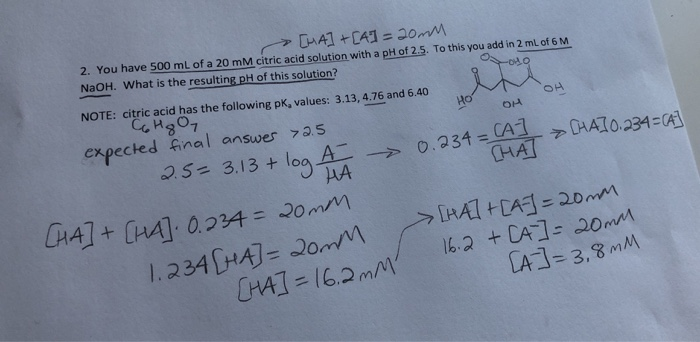





![Solved 6 SOT 4. a) [2] One litre of buffer is made by mixing | Chegg.com Solved 6 SOT 4. a) [2] One litre of buffer is made by mixing | Chegg.com](https://media.cheggcdn.com/study/e68/e687f593-aceb-416b-bd35-b4e070da579b/image.png)