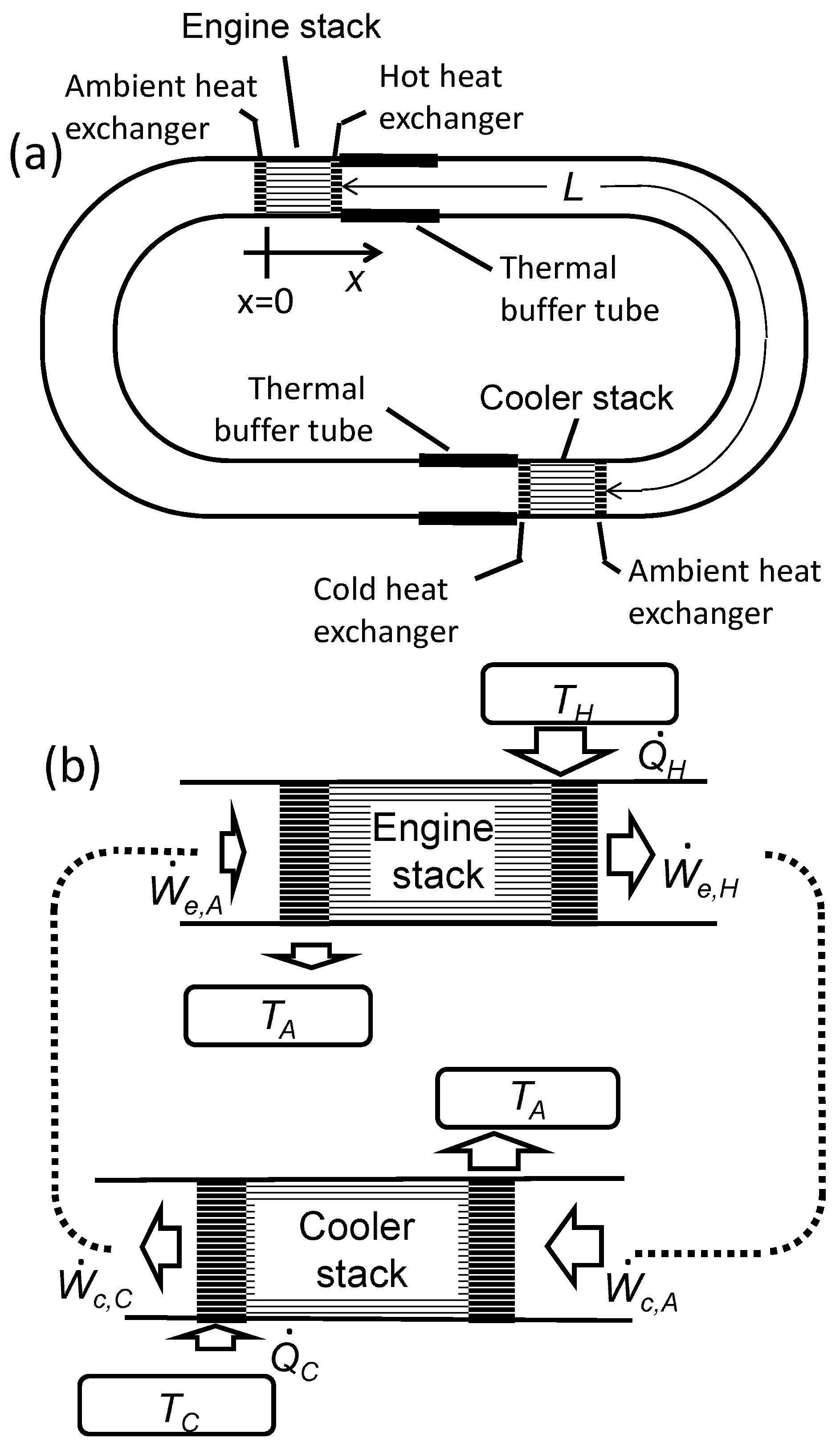
Calculate the difference between the two specific heats of Helium gas per gram. Given that molecular weight of He = 4, J = 4.18 joule/cal and R = 8.31 J mole^-1//K^-1?

SOLVED: A heat engine using 140 mg of helium as the working substance follows the cycle shown in the figure igure 1) Figure 1 of 1 (atm) Isotherm (cm ) Vnnx 1000

15.14 | Calculate the net work output of a heat engine following path ABCDA in the figure below. - YouTube

The operation of a certain heat engine takes an ideal monatomic gas through a cycle shown as the rectangle on the PV diagram below. a) Determine the efficiency of this engine. Let
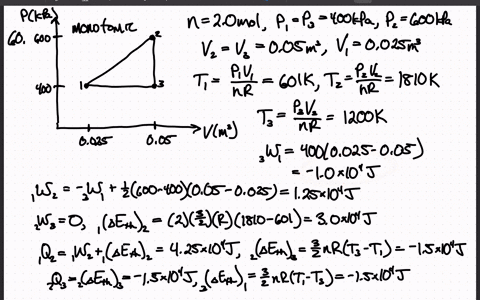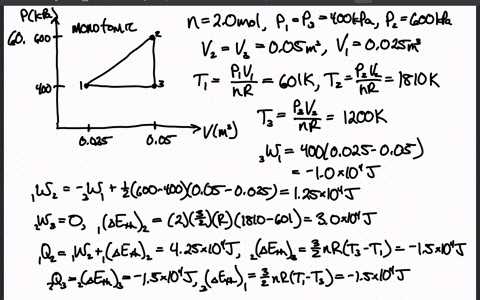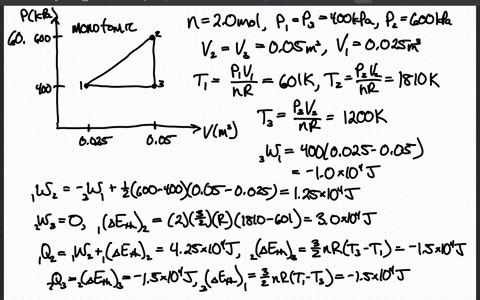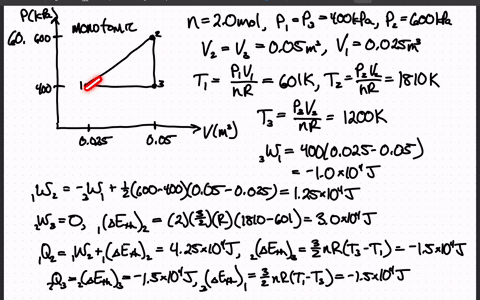
SOLVED:The heat engine shown in HGURE P19.60 uses 2.0 mol of a monatomic gas as the working substance. a. Determine T1, T2, and T3 b. Make a table that shows ΔEth, Ws,
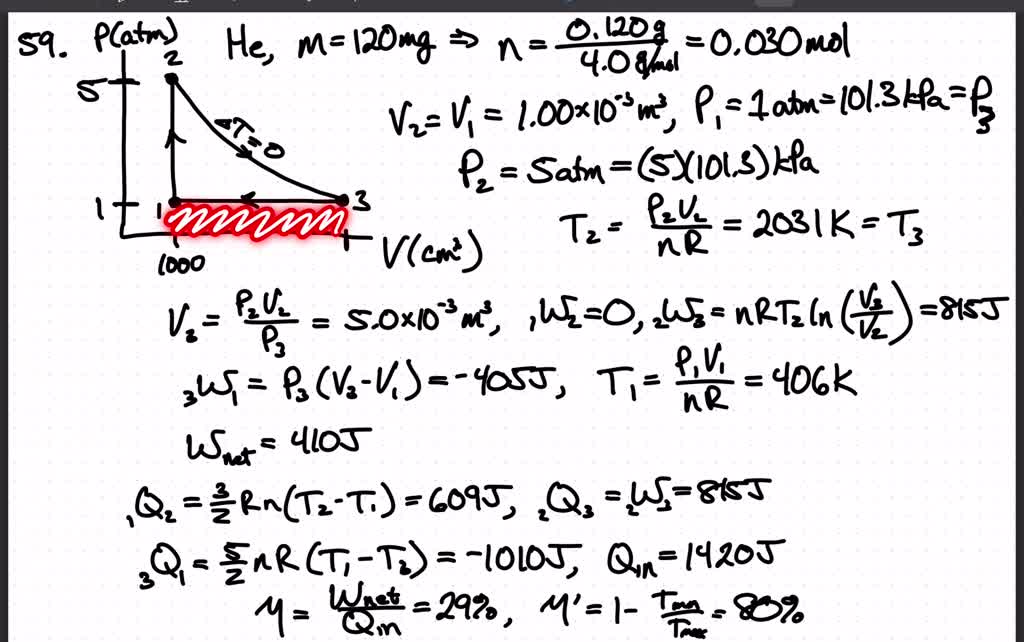
SOLVED:A heat engine using 120 mg of helium as the working substance follows the cycle shown in FIGURE P19.59. a. Determine the pressure, temperature, and volume of the gas at points 1,2,
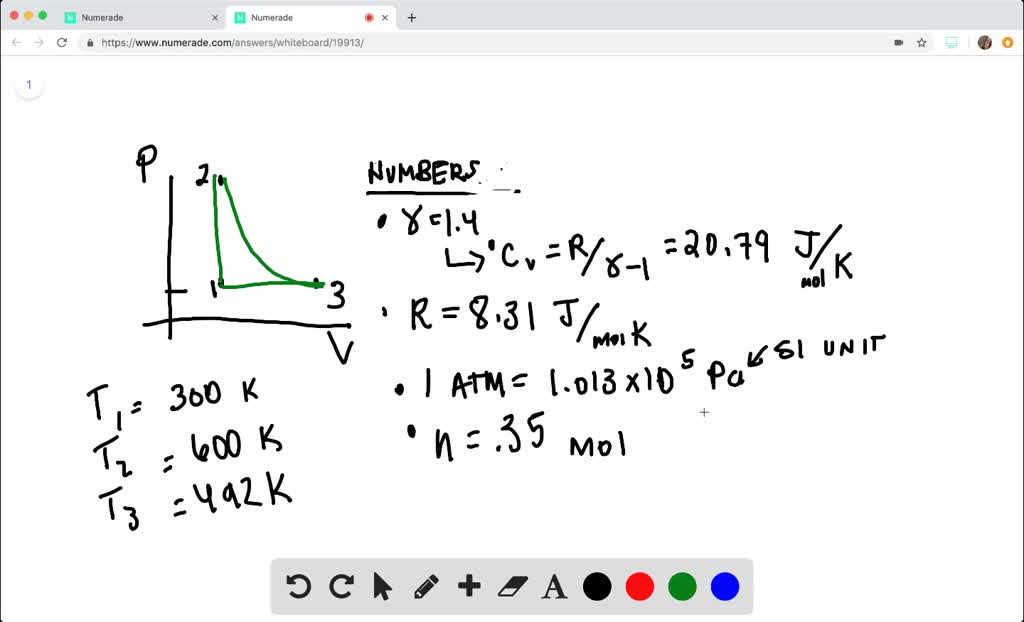
SOLVED:A heat engine takes 0.350 mol of a diatomic ideal gas around the cycle shown in the pV-diagram of Fig. P20.36. Process 1→2 is at constant volume, process 2→3 is adiabatic, and