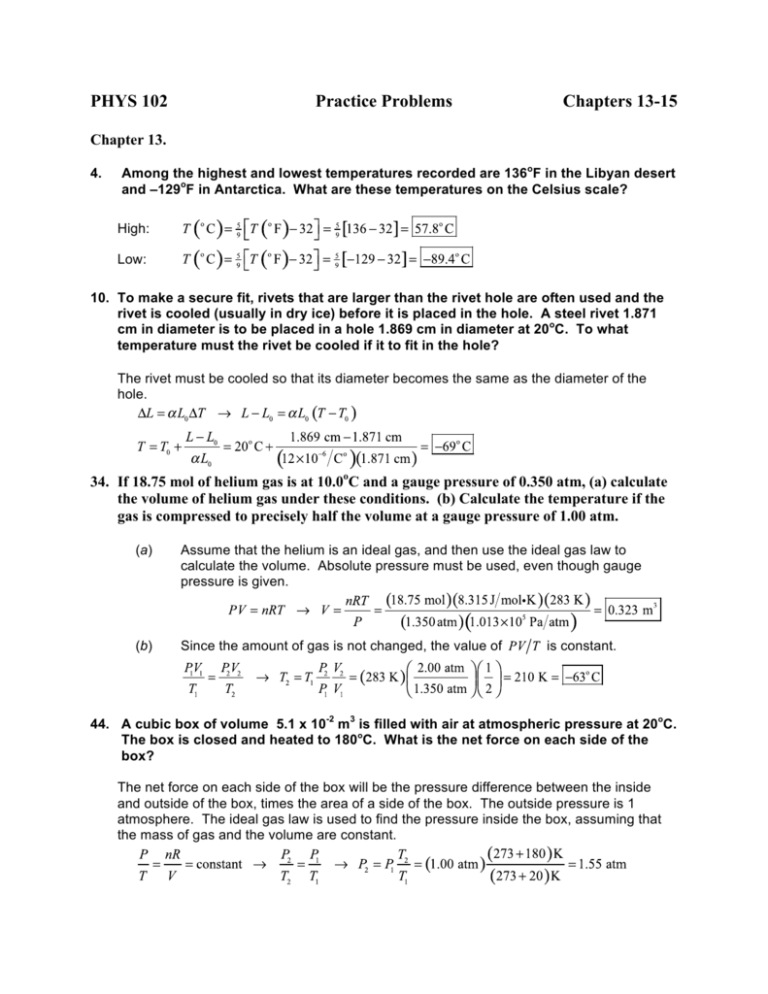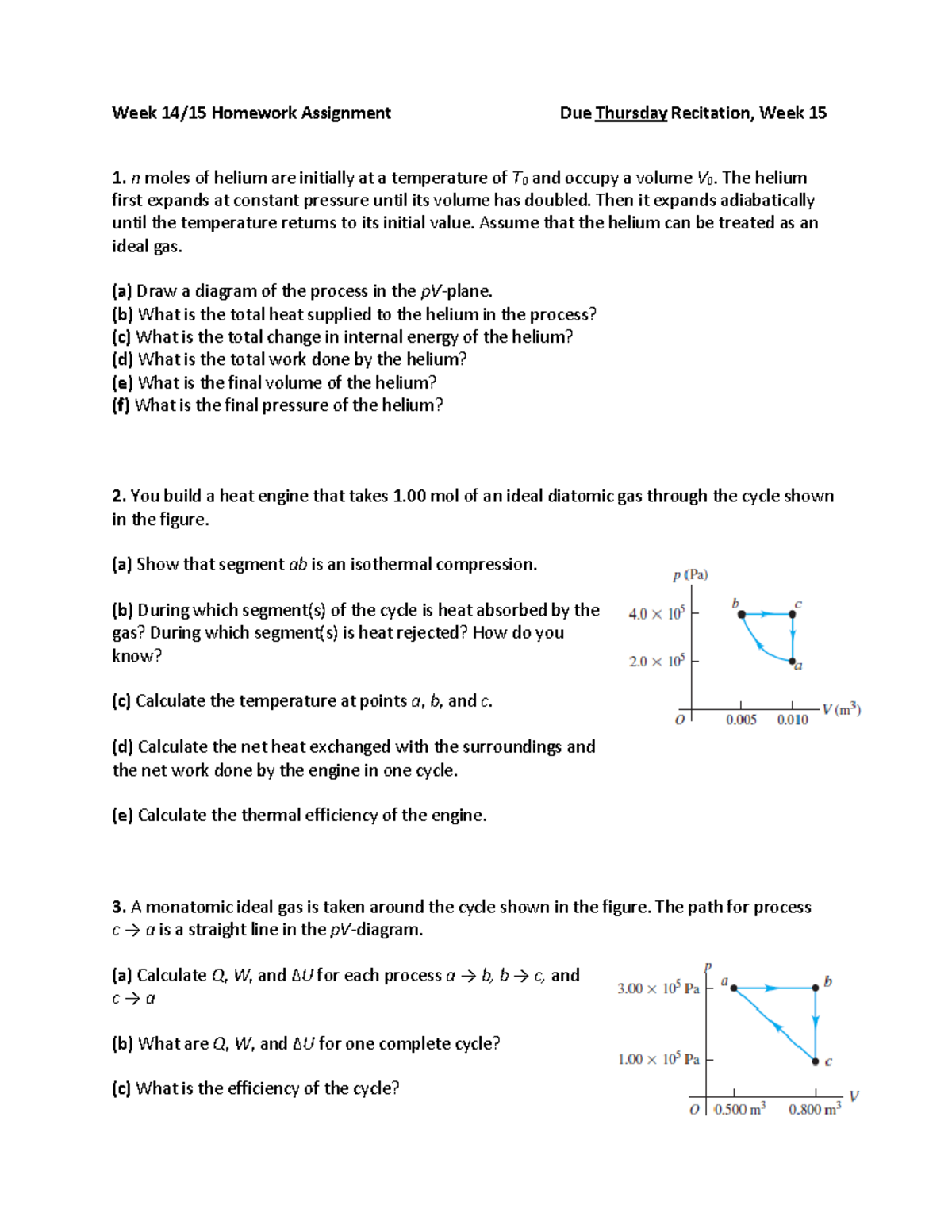
Week 14 Homework Assignment - n moles of helium are initially at a temperature of T0 and occupy a - Studocu

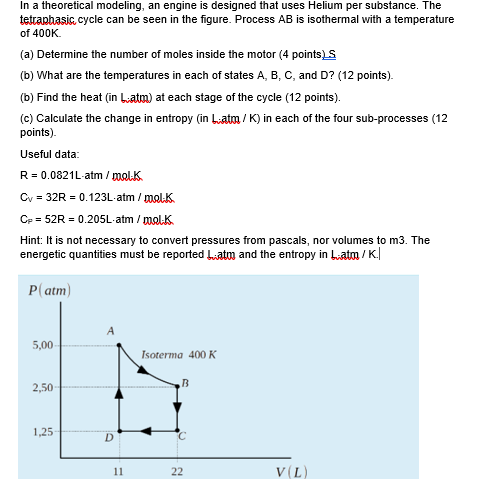
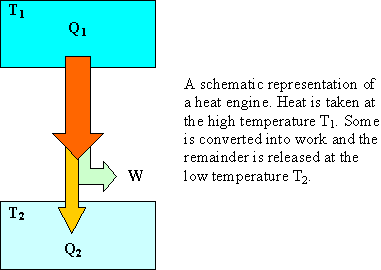
A heat engine operates in the reversible cycle shown in the PV diagram below. The temperature of state A is 300 K. Process BC is isothermal. The engine contains 0.5 moles of
Entropy | Free Full-Text | Performance Analysis and Four-Objective Optimization of an Irreversible Rectangular Cycle
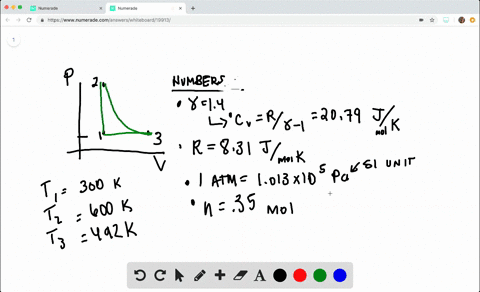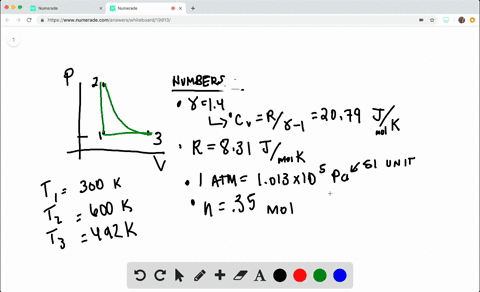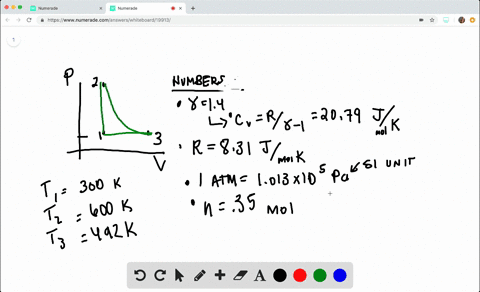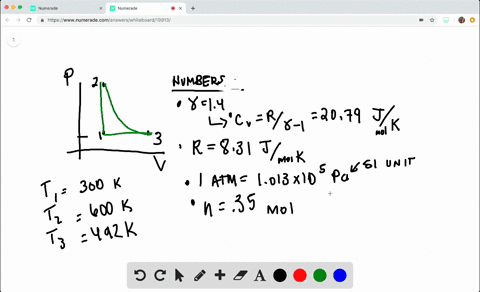
SOLVED:A heat engine takes 0.350 mol of a diatomic ideal gas around the cycle shown in the pV-diagram of Fig. P20.36. Process 1→2 is at constant volume, process 2→3 is adiabatic, and
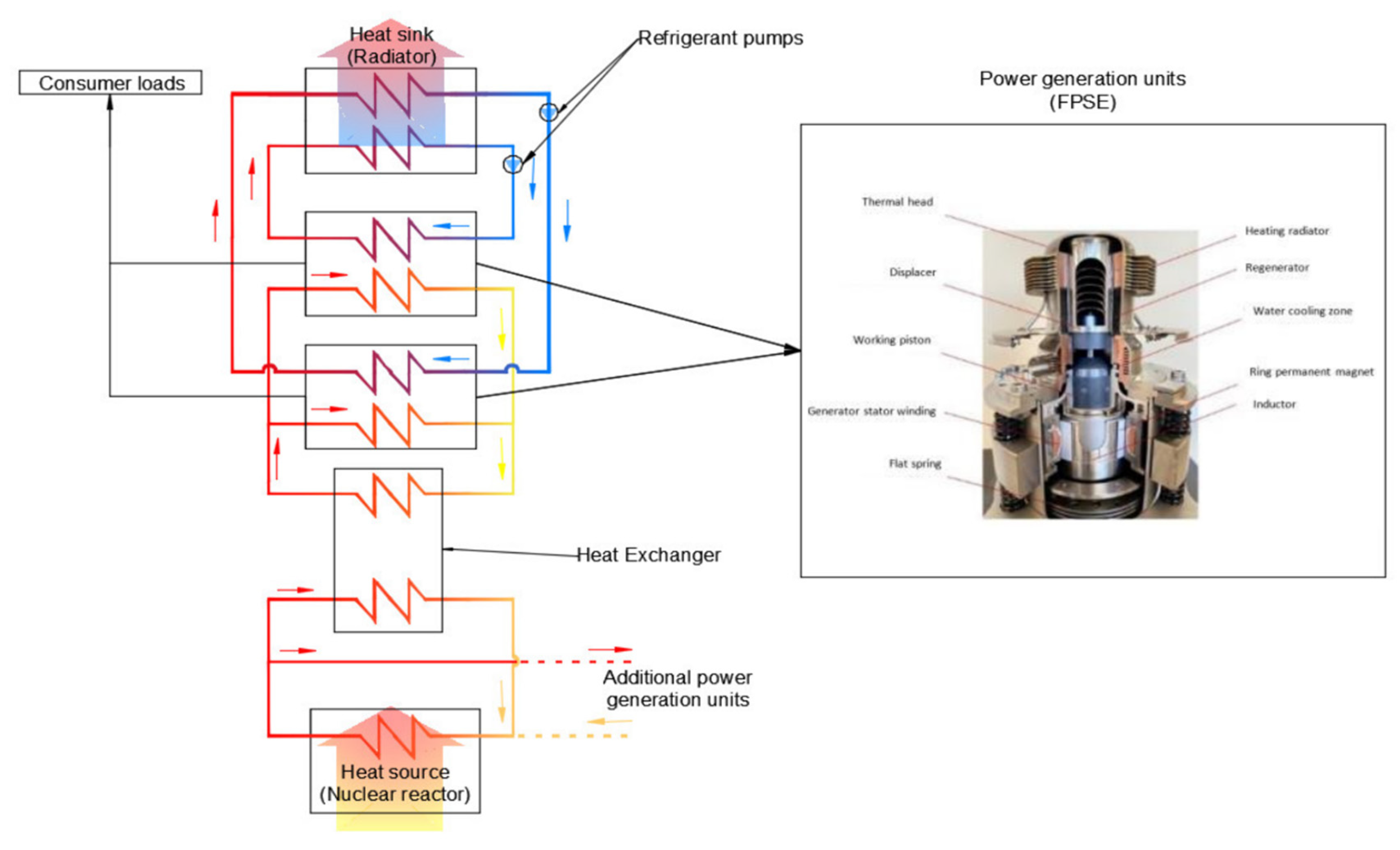
Symmetry | Free Full-Text | An Enhanced Calculation Method of the Heat Rejection System of a Free-Piston Stirling Engine (FPSE) Operating on the Moon

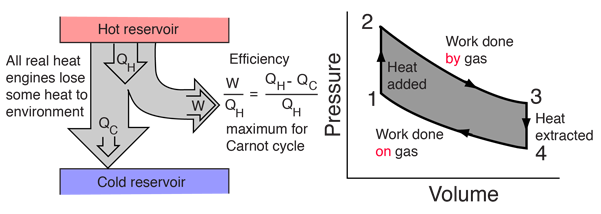
Heat Engines, Thermal Efficiency, & Energy Flow Diagrams - Thermodynamics & Physics Problems - YouTube
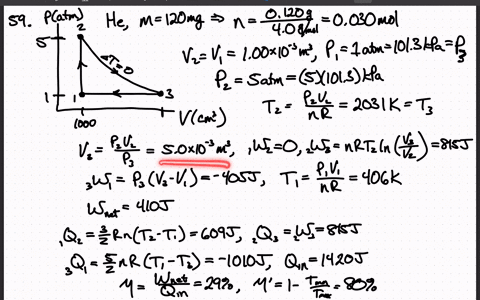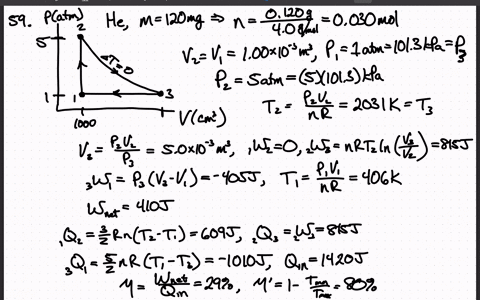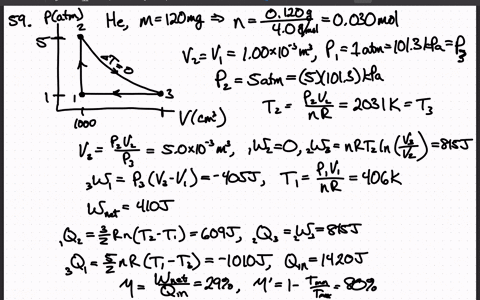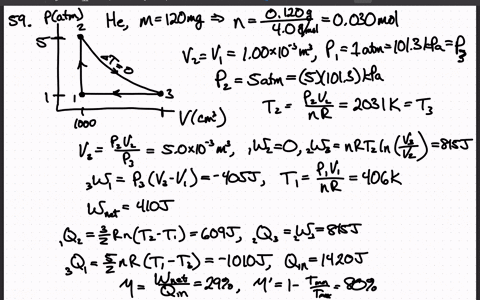
SOLVED:A heat engine uses 100 . mg of helium gas and follows the cycle shown in the figure. a) Determine the pressure, volume, and temperature of the gas at points 1 ,

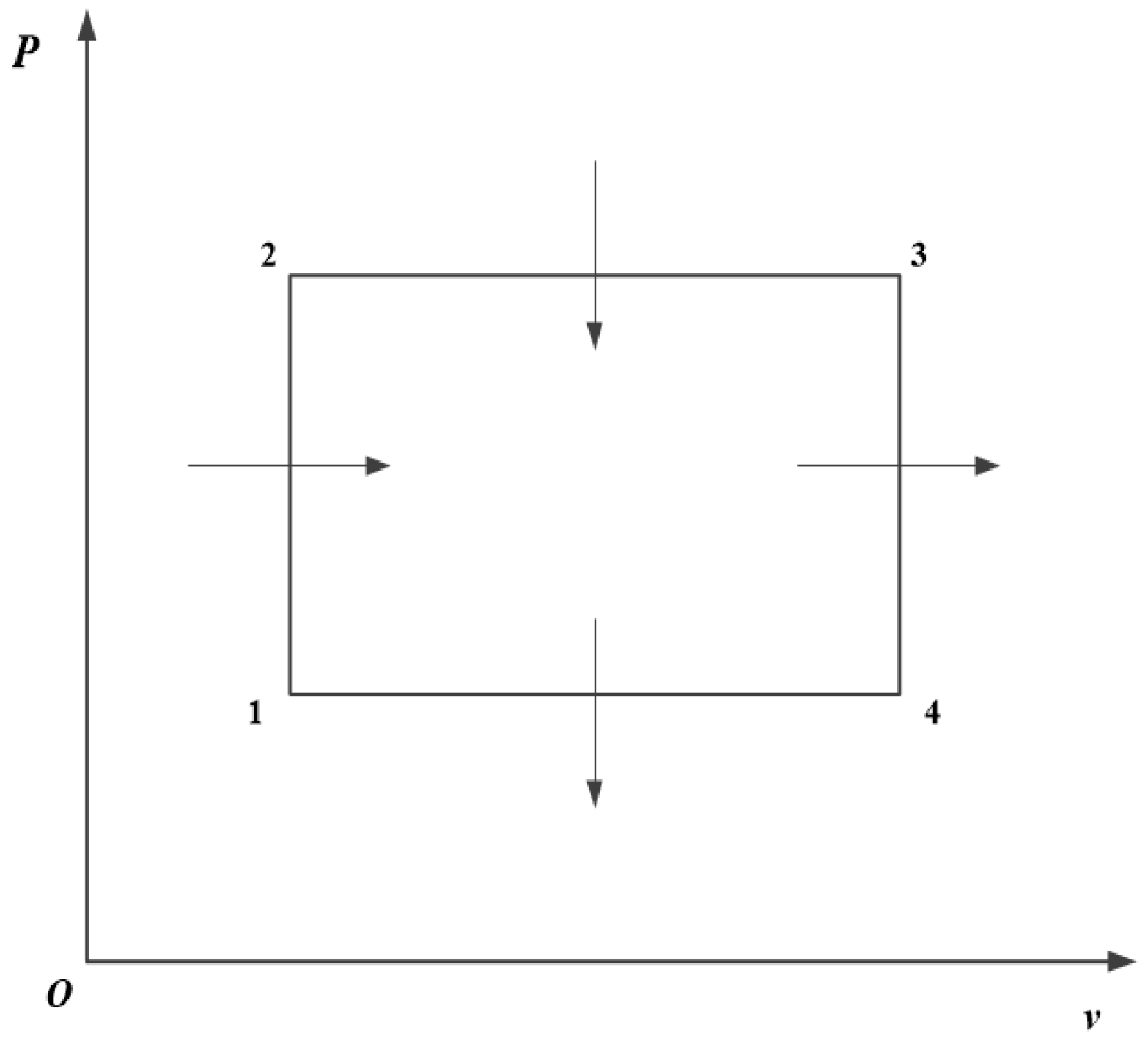
The operation of a certain heat engine takes an ideal monatomic gas through a cycle shown as the rectangle on the PV diagram below. a) Determine the efficiency of this engine. Let
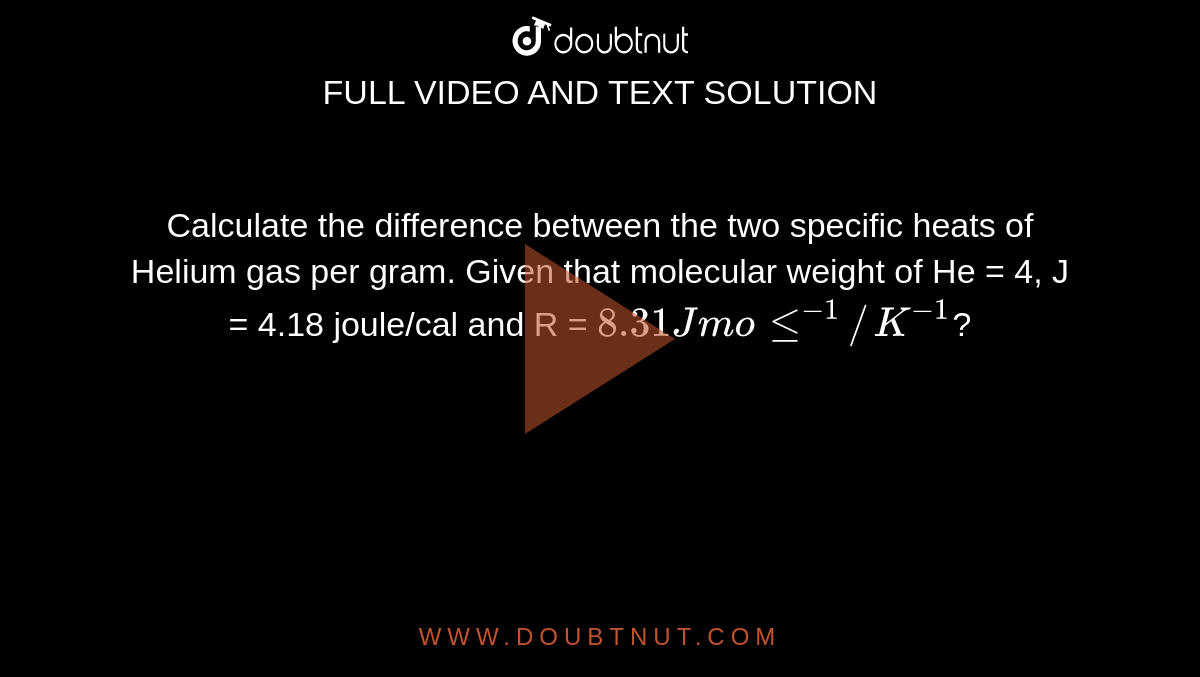
Calculate the difference between the two specific heats of Helium gas per gram. Given that molecular weight of He = 4, J = 4.18 joule/cal and R = 8.31 J mole^-1//K^-1?
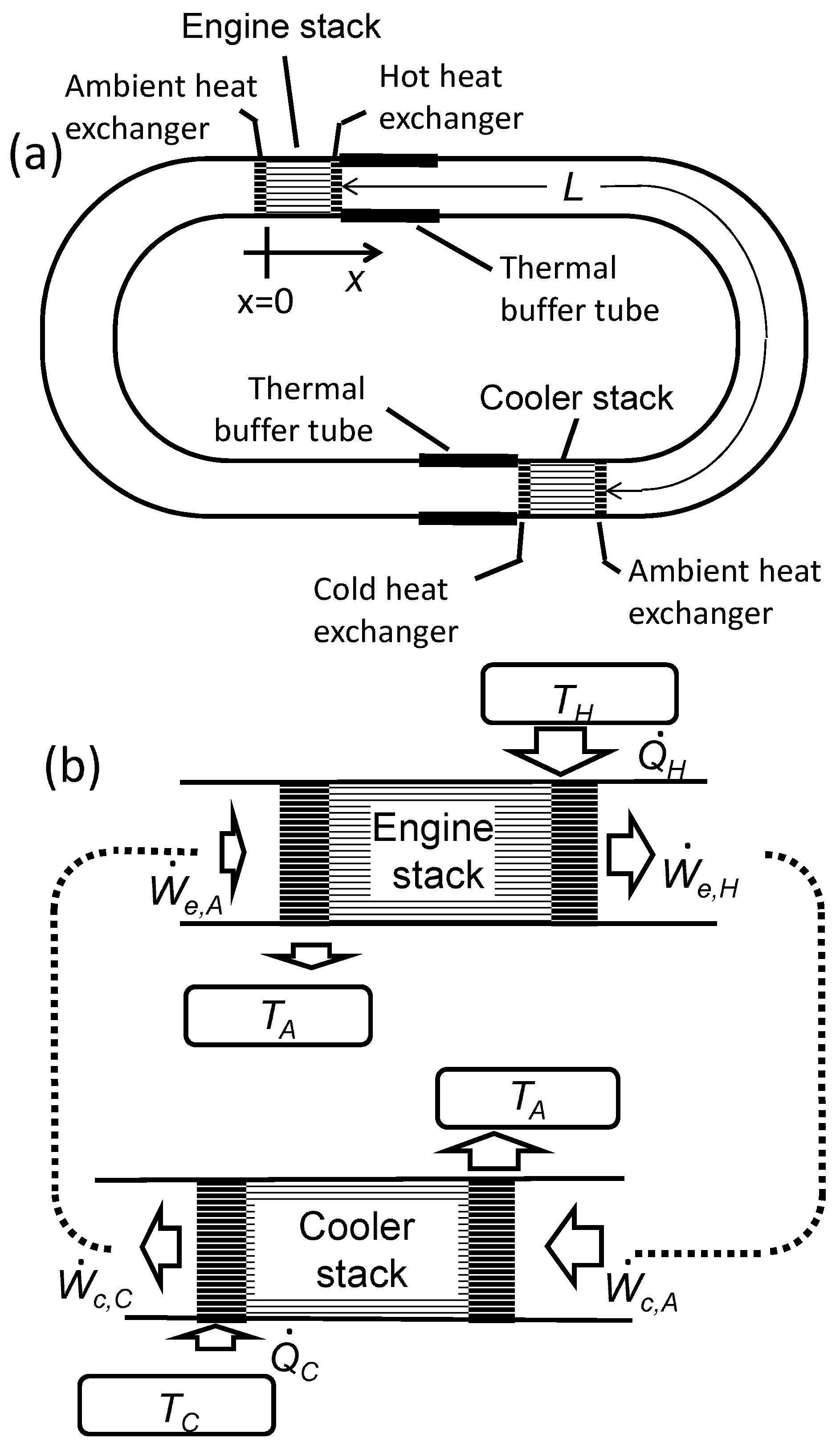
Applied Sciences | Free Full-Text | Numerical Calculation of the Performance of a Thermoacoustic System with Engine and Cooler Stacks in a Looped Tube